Study Reveals Targeted Therapy for Aggressive Liver Cancer
Combining angiogenesis inhibitors with immune checkpoint inhibitors shows promise in treating aggressive liver tumors
Hepatocellular carcinoma (HCC), the most common liver cancer, includes aggressive subtypes resistant to treatment. Researchers from Institute of Science Tokyo, Japan, identified a key mechanism behind one such subtype and developed a mouse model replicating its characteristics. Their study found that combining angiogenesis inhibitors with immune checkpoint blockers effectively disrupted the tumor’s defenses, enabling immune cells to attack. These findings offer a promising strategy for improving treatments and outcomes for patients with aggressive HCC.
Targeting Aggressive Hepatocellular Carcinoma:
A Combined Anti-Angiogenesis and Immunotherapy Approach
Taniai et al. (2025) | Hepatology | DOI:10.1097/HEP.0000000000001284
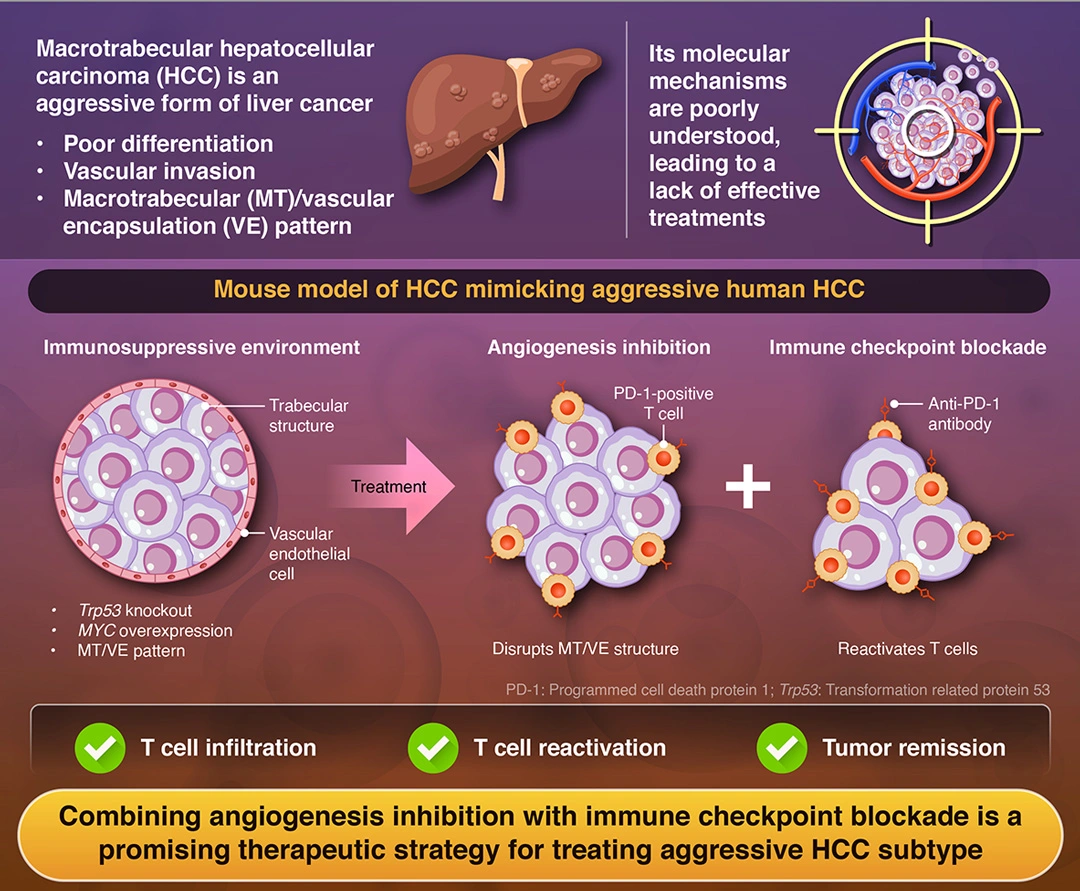
Among the many forms of liver cancer, hepatocellular carcinoma (HCC) is the most common and one of the leading causes of cancer-related deaths worldwide. It is a complex disease with multiple aggressive subtypes that are difficult to treat. One particularly aggressive variant is characterized by tumors that form large trabecular (layered) structures around blood vessels, effectively encapsulating them. This macrotubular (MT)/vascular encapsulation (VE) pattern creates a microenvironment that not only promotes tumor growth but is also immunosuppressive and resistant to standard treatments.
A recent study published online in Hepatology on February 26, 2025, brings new hope. A research team led by Professor Shinji Tanaka, along with Assistant Professor Shu Shimada from Institute of Science Tokyo (Science Tokyo), Japan, found that targeting angiogenesis alongside immune checkpoint blockade can disrupt the tumor’s protective structure, enabling immune cells to infiltrate and attack the cancer.
“Using integrated analysis of large-scale bulk and single-cell RNA-sequencing datasets, we refined the molecular and immunological classification of liver cancer. Utilizing these findings, we developed a syngeneic mouse model of aggressive HCC, which exhibited highly mitotic, tumorigenic, and metastatic abilities with the MT/VE structure contributing to immune evasion,” explains Tanaka.
To develop the treatment, the researchers first had to determine why and how these tumors develop and form the MT/VE structure. Cancer cells often exhibit distinct gene expression profiles compared to normal cells, with certain genes being upregulated (overexpressed) or downregulated (underexpressed). Using RNA sequencing, researchers analyze these abnormal patterns to develop targeted therapies that block cancer-promoting processes.
The researchers analyzed over 228,000 individual cells using single-cell RNA sequencing and combined this with data from 691 HCC tumor samples. They found that the aggressive HCC subtype, classified as molecular subtype 1 (MS1), was characterized by a combination of rapidly dividing cells with high mitotic activity and metabolically active cells with elevated glucose metabolism or fat synthesis.
This subtype was characterized by TP53 mutations, overactive MYC signaling, and fewer T cells. TP53 is a gene that encodes the tumor-suppressor protein (p53), which prevents cancer, but mutations disable this function. MYC is an oncogene that drives cell growth and tumor progression, while the low number of T cells weakens the immune system’s ability to fight the tumor. Together, these factors make the cancer more aggressive and harder to treat.
To study potential treatments, the researchers developed a mouse model of HCC that mimicked these characteristics. They injected mice with liver cancer cells lacking the Trp53 gene and overexpressing MYC. These tumors exhibited cellular and histopathological characteristics similar to the MS1 subtype. The mouse model HCC was susceptible to a combination of an angiogenesis inhibitor and an anti-PD-1 antibody, an immune checkpoint inhibitor.
Treatment with angiogenesis inhibitors blocked the formation of new blood vessels, while the immune checkpoint inhibitors reactivated T cells, enabling them to attack the cancerous cells. “For MT/VETC-type liver cancer, combined therapy using potent anti-angiogenic agents and immune checkpoint inhibitors resulted in significant tumor shrinkage, suggesting that this novel therapeutic strategy may be effective,” says Tanaka.
The results of this study offer a promising path toward improving survival and quality of life for patients with this challenging disease.
Reference
- Authors:
- Tomohiko Taniai,1,2 Shu Shimada,1 Yoshimitsu Akiyama,1 Megumi Hatano,1 Koya Yasukawa,1 Yosuke Igarashi,1,2 Shu Tsukihara,1,2 Yoshiaki Tanji,1,2 Keita Kodera,1,2 Kohei Okazaki,1,2 Koichiro Haruki,2 Atsushi Nara,1,3 Kohei Yagi,1,3 Keiichi Akahoshi,3 Daisuke Ban,3 Yasuhiro Asahina,4 Toru Ikegami,2 Minoru Tanabe,3 Shinji Tanaka1,3
- Title:
- Integrative transcriptome profiling elucidates molecular and immunovascular
characteristics of macrotrabecular hepatocellular carcinoma - Journal:
- Hepatology
- Affiliations:
- 1Department of Molecular Oncology, Graduate School of Medical and Dental Sciences, Institute of Science Tokyo, Japan
2Department of Surgery, The Jikei University School of Medicine, Japan
3Department of Hepato-Biliary-Pancreatic Surgery, Graduate School of Medicine, Institute of Science Tokyo, Japan
4Department of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Institute of Science Tokyo, Japan
Related articles
Further information
Professor Shinji Tanaka
Department of Molecular Oncology, Graduate School of Medical and Dental Sciences, Institute of Science Tokyo
- tanaka.monc@tmd.ac.jp
Assistant Professor Shu Shimada
Department of Molecular Oncology, Graduate School of Medical and Dental Sciences, Institute of Science Tokyo
- shimada.monc@tmd.ac.jp
Contact
Public Relations Division, Institute of Science Tokyo
- Tel
- +81-3-5734-2975
- media@adm.isct.ac.jp