Nano-Patterned Copper Oxide Sensor for Ultra-Low Hydrogen Detection
Researchers have developed highly reliable and fast-responding hydrogen sensors to meet the growing needs of the hydrogen industry
A novel hydrogen sensor developed by researchers at Institute of Science Tokyo offers a promising solution for real-time hydrogen leak detection, addressing safety concerns in industrial applications. This sensor, made with nano-patterned cupric-oxide (CuO) nanowires (NWs) with voids, can detect hydrogen at extremely low concentrations with high response, recovery speed, and precision, significantly improving previous CuO-based sensors. It has the potential to enable safer and more reliable use of hydrogen in clean energy applications.
Highly Sensitive Cupric Oxide Gas Sensor for Hydrogen Leak Detection
Zhao et al. (2024) | Advanced Functional Materials | 10.1002/adfm.202415971
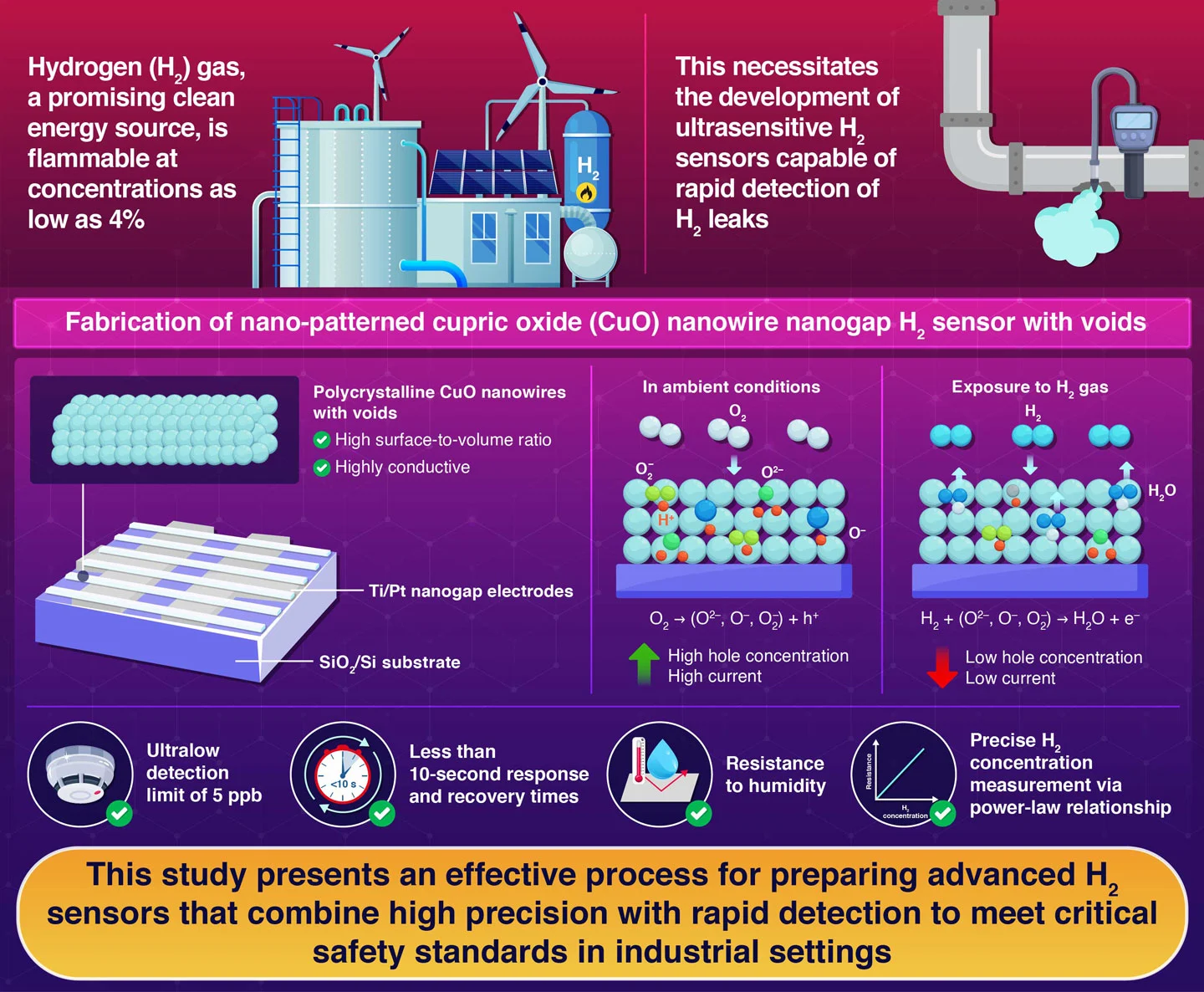
Hydrogen is becoming an increasingly popular choice as we shift towards cleaner energy. It can be burned like traditional fuels, producing only water as a byproduct, and can generate electricity when used in fuel cells. However, as hydrogen production, use, and transportation increase, so do safety concerns. Hydrogen is highly flammable at concentrations as low as 4% and is odorless and colorless, making leaks challenging to detect.
To address these concerns, researchers led by Professor Yutaka Majima from Institute of Science Tokyo (Science Tokyo) have developed a sensor that detects hydrogen at ultra-low concentrations with a very short response time. Their study was published in the journal Advanced Functional Materials on November 5, 2024.
The sensor is made from nano-patterned polycrystalline CuO NWs, which are highly sensitive to hydrogen gas, placed on a silicon (SiO2/Si) substrate with platinum/titanium electrodes. “We employed electron-beam lithography and two-step ex-situ oxidization to develop a reliable and reproducible process for preparing high-performance, nano-patterned CuO nanowire-nanogap hydrogen gas sensors with voids, which is considerably different from conventional free-standing single-crystal CuO nanowires directly grown from copper sources,” says Prof. Majima.
When exposed to hydrogen gas, the sensor operates by detecting changes in the electrical resistance of CuO NWs. In air, oxygen molecules attach to the surface of the CuO NWs, forming oxygen ions (O2-, O-, O22-) that induce a layer of positive charge carriers (holes) near the surface. When hydrogen is introduced, it reacts with the oxygen ions on the surface of the CuO NWs to form water, which lowers the hole concentration. As a result, the NWs become less conductive. By measuring the increase in resistance, the sensor can detect the presence and concentration of hydrogen gas.
The researchers enhanced the sensor's performance by introducing a pre-annealing step in a hydrogen-rich environment, followed by slow oxidation in dry air. Initially, the fabricated copper (Cu) NWs have low crystallinity and form a Cu oxide layer on the surface, hindering interaction with oxygen. The annealing process reshapes the Cu NWs from a rectangular to a semicircular arch form, improving their crystallinity. In the subsequent oxidation step, the Cu NWs are converted into copper oxide. During this process, copper atoms diffuse outward to react with oxygen, creating voids that increase the surface area of the NWs, providing more active sites for hydrogen and oxygen to interact with the NW.
As a result of these improvements, the sensor can detect hydrogen concentrations as low as 5 parts per billion (ppb), much lower than previous CuO-based H2 sensors. Additionally, it is resistant to humidity, a common drawback of CuO gas sensors. The sensor also responds quickly, detecting hydrogen in just 7 seconds.
The researchers further enhanced the sensor’s performance by reducing the nanogap separation between the electrodes. A smaller gap generates a stronger electric field, accelerating the movement of charge carriers and speeding up the sensor’s response and recovery. With a gap size of 33 nm, the sensor detected 1,000 ppm of H2 in just 5 seconds and returned to baseline conditions in 10 seconds. “We will continue developing a wider range of gas sensors with this process to fabricate sensors for other hazardous gases as well,” says Prof. Majima.
By early detection of leaks or unsafe gas levels, the sensor can help mitigate risks and enable the safe adoption of hydrogen technologies, supporting the transition to a hydrogen-based economy.
Reference
- Authors:
- Muqing Zhao1, Ryosuke Nitta1, Seiichiro Izawa1, Jun-ichi Yamaura2, and Yutaka Majima1*
- Title:
- Nano-Patterned CuO Nanowire Nanogap Hydrogen Gas Sensor with Voids
- Journal:
- Advanced Functional Materials
- Affiliations:
- 1 Materials and Structures Laboratory, Institute of Integrated Research, Institute of Science Tokyo, Japan
2 Institute of Solid-State Physics, The University of Tokyo, Japan
Related articles
Further information
Professor Yutaka Majima
Institute of Integrated Research, Institute of Science Tokyo
- majima@msl.titech.ac.jp
Contact
Public Relations Division, Institute of Science Tokyo
- media@adm.isct.ac.jp
- Tel
- +81-3-5734-2975