Glowing under pressure: hinge-like mechanophores for smarter polymeric materials
Researchers develop hinge-like molecules using [2.2] paracyclophane, which illuminates in response to mechanical stress
In a step toward smarter materials, researchers from Institute of Science Tokyo collaborated with researchers from Switzerland to develop a smart hinge-like molecule that can indicate mechanical stress in polymeric materials through fluorescence. Using a framework of [2.2]paracyclophane and two pyrene-based luminophores (light-emitting compounds), the developed molecule exhibits excellent stress-sensing with high durability—offering a powerful tool for real-time monitoring of mechanical damage.
Hinge-Like Mechanophores: Visualizing Stress with a Molecular Twist
Sagara et al. (2025) | Angewandte Chemie International Edition | 10.1002/anie.202510114
Flexible polymers like elastomers are used everywhere—from cushions in shoes and furniture to car bumpers and medical devices. However, when such materials undergo excessive mechanical stress, the damage often starts at the molecular level long before it is visible on the surface. Evaluating this damage is critical to ensure the durability of such materials. This requirement drove the invention of mechanochromic mechanophores—molecular frameworks that can respond to mechanical stimuli with a change in luminescence color.
Delving deeper into this, a group of researchers led by Associate Professor Yoshimitsu Sagara of the School of Materials and Chemical Technology, Institute of Science Tokyo (Science Tokyo), Japan, and Professor Christoph Weder of the Adolphe Merkle Institute, University of Fribourg, Switzerland, have developed a new class of mechanochromic mechanophores for visualizing mechanical force by skillfully utilizing [2.2]paracyclophane. The findings of the developed system were published online in Angewandte Chemie International Edition on June 30, 2025.
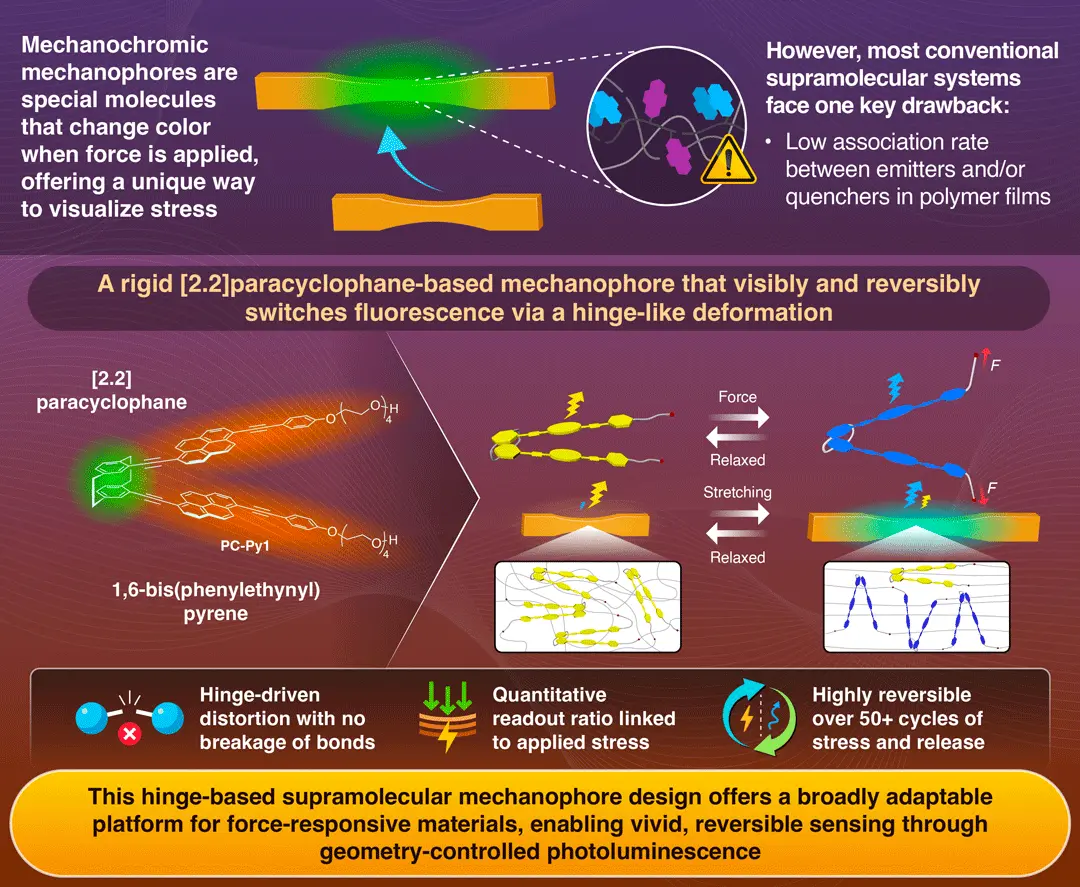
Traditionally, mechanical sensing largely relied on either excimer-forming dye doping or covalently introducing mechanophores whose working mechanisms rely on breakage of covalent bonds. While dye doping offered reversible sensing, its mechano-responsive behavior was strongly dependent on the nature of the polymer and processing conditions. Whereas, covalent mechanophores indicated signals at the molecular level, but the cleavage of covalent bonds made it irreversible. To overcome these limitations, supramolecular mechanophores were introduced. These molecules exhibit mechano-responsive behavior at the molecular level but with non-covalent interactions.
“Many supramolecular mechanophores exist, but some of them show inefficient signaling,” explains Sagara, “We aimed to develop a system that emits more efficient and reliable signals in direct proportion to the applied stress—and does so reversibly.”
[2.2]Paracyclophane is an organic compound in which two benzene rings are connected by two ethylene bridges at the para positions. The team used [2.2]paracyclophane as a backbone and introduced two fluorescent groups (pyrene-based dyes) to form a hinge-like structure called PC-Py1. The two pyrene-based dyes were forcibly held in close proximity, enabling efficient formation of excimers (emitter dimers formed when two molecules bind together in an excited state). This resulted in bright yellow fluorescence by excimers. The framework was then embedded in flexible polyurethane elastomer for analysis.
When mechanical force was applied to the PC-Py1 through polymer chains, the fluorescent groups separated, releasing the forced proximity. This change results in a fluorescence shift, changing the fluorescence color from yellow (excimer state) to blue-green (monomer state).
“The fluorescence color change is not only clearly visible to the eye but also reversible. What’s more, it closely tracks the actual mechanical stress,” remarks Sagara.
The key factor was the structural rigidity of the [2.2]paracyclophane molecular skeleton. When more flexible versions of the molecule were tested, they produced weaker color shifts. Additionally, the hinge-like motion of the system acted as the source of the signal, which was confirmed through mechanical and spectroscopic studies. Moreover, the mechanophore remarkably endured more than 50 cycles of stress and release—confirming the durability and stability of the hinge mechanism.
The study marks a significant advance in the design of supramolecular mechanophores. By leveraging the forced proximity effect of [2.2]paracyclophane, it is possible to incorporate weakly interacting fluorescent or quenching groups into mechanoresponsive systems— components previously considered unsuitable. This strategy expands the design flexibility and photo-functional diversity of such materials, offering a powerful new platform for visualizing mechanical force.
The findings of this study not only deepen our understanding of mechanophores but also open doors to various practical applications ranging from damage-sensing coatings and flexible electronics to smart wearable technologies. Additionally, by tuning the molecular design, the strategy could further utilize a wide range of fluorophores—paving the way for next-generation customizable stress sensing.
Reference
- Authors:
- Shohei Shimizu1, Jess M. Clough2,3, Christoph Weder2,3, and Yoshimitsu Sagara1,4*
- Title:
- Hinge-Like Mechanochromic Mechanophores Based on [2.2]Paracyclophane
- Journal:
- Angewandte Chemie International Edition
- Affiliations:
- 1Department of Materials Science and Engineering, Institute of Science Tokyo, Japan
2Adolphe Merkle Institute, University of Fribourg, Switzerland
3NCCR Bio-inspired Materials, University of Fribourg, Switzerland
4Research Center for Autonomous Systems Materialogy (ASMat), Institute of Integrated Research, Institute of Science Tokyo, Japan
Related articles
Further Information
Associate Professor Yoshimitsu Sagara
Department of Materials Science and Engineering, Institute of Science Tokyo
- sagara@mct.isct.ac.jp
Contact
Public Relations Division, Institute of Science Tokyo
- Tel
- +81-3-5734-2975
- media@adm.isct.ac.jp