How a common immunosuppressive drug "ATG" injures liver blood vessels
Human organoid model uncovers a two-step mechanism for ATG-induced liver injury
Leveraging human organoid-based mechanistic investigation, researchers reveal how an immunosuppressive drug, antithymocyte globulin (ATG), induces injury to blood vessels in the liver. According to the study, ATG first triggers rapid clotting through a complement activation system and later causes inflammation by activating the TGF-β pathway. This discovery explains why some patients experience severe liver-related side effects following organ transplantation and aid in developing safer immunosuppressive regimen.
How Antithymocyte Globulin (ATG) Causes Liver Microvascular Injury in Humans
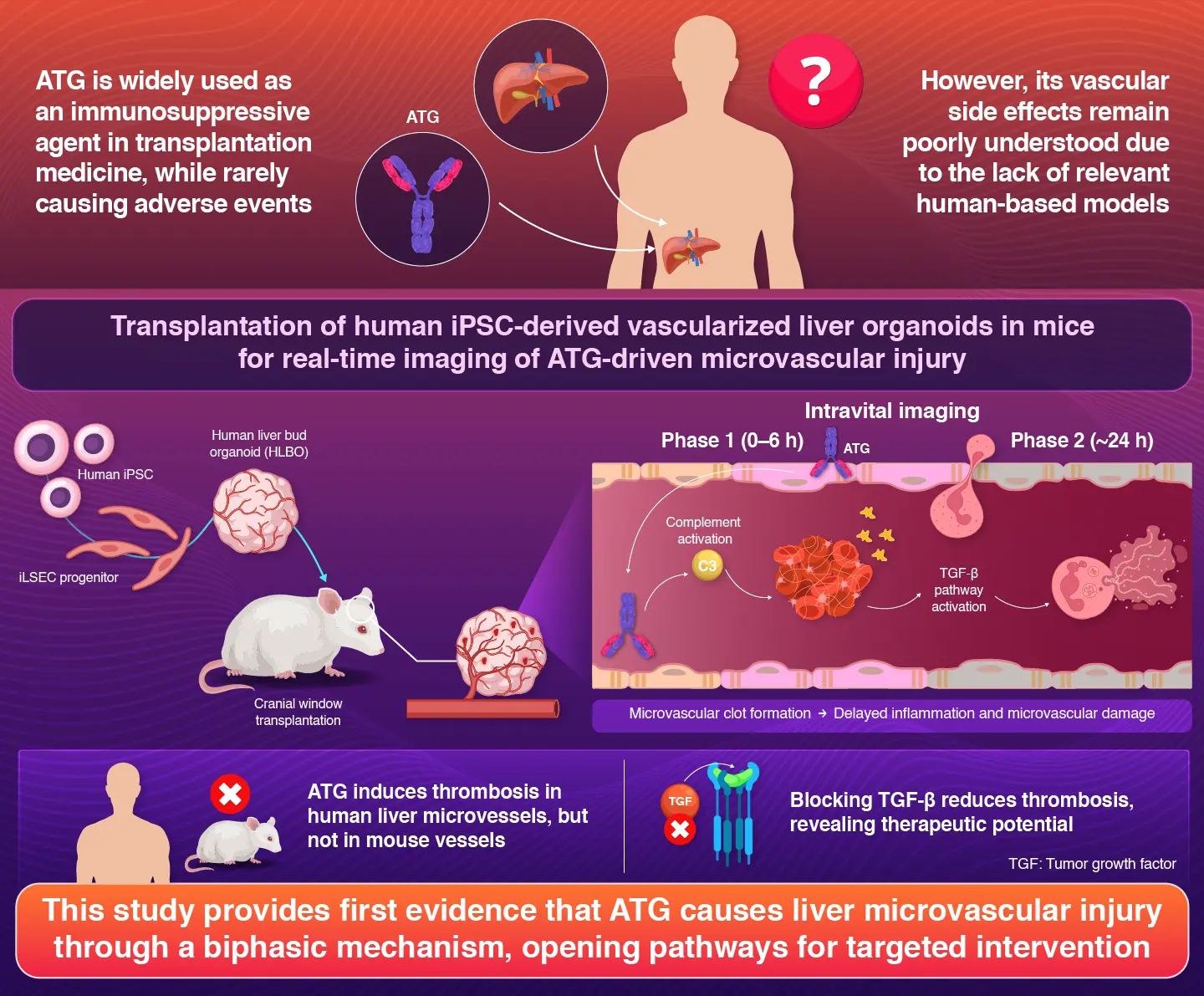
Immunosuppressants are medications that suppress the immune system. These are particularly essential for patients undergoing organ transplantation, as they help prevent the body's immune system from rejecting the donated organ. One such drug is antithymocyte globulin (ATG), which is widely used to prevent organ rejection in patients. However, ATG is known to cause serious side effects like blood clots and impaired liver function. The reason behind these side effects was not fully understood, until now.
To shed light on this, a research team at Institute of Science Tokyo (Science Tokyo), Japan, led by Professor Takanori Takebe and doctoral student Shuntaro Kawamura, from Human Biology Research Unit, Institute of Integrated Research at Science Tokyo, along with Assistant Professor Noriki Okada from Jichi Medical University, Japan, developed a novel organoid-based imaging system for real-time monitoring of the ATG-induced effects. Their findings were made available online on November 6, 2025, and published in Volume 6, Issue 11 of the journal Cell Reports Medicine on November 18, 2025.
"Using human induced pluripotent (iPS) cells, we first developed a human organoid model which acted like a simplified version of human liver containing its own network of microvasculatures," explains Takebe.
By transplanting these tiny organoids into mice and leveraging artificial intelligence-based imaging analysis, the researchers achieved a unique high-resolution intravital imaging system that enabled live monitoring of human vascular function in living tissue. The team then exposed this model to ATG to see exactly how the drug affects the hepatic microvasculature, formed by iPS cell-derived liver sinusoidal endothelial cells (iLSECs).
The researchers discovered that ATG injures hepatic blood vessels via a biphasic mechanism. The first step happens quickly. In this step, the ATG binds to iLSECs, triggering an immune response called complement activation. This causes platelets to clump together, leading to the formation of blood clots within a few hours.
The second step develops more slowly, occurring almost 24 hours after exposure. In this phase, ATG activates a TGF-β signaling pathway of iLSECs, leading to a proinflammatory and prothrombotic program. In the same period, intravital microscopy exhibited accumulating neutrophils releasing enzymes that further worsen endothelial damage. The combination of these two steps was suggested to disrupt the blood flow in the liver, leading to severe side effects experienced by patients who underwent liver transplantation.
To understand how these findings relate to real patients, the researchers also examined liver tissues from patients who had developed liver complications after receiving ATG. The obtained samples showed similar biological signs as observed in the organoid model, confirming the complement proteins, platelet-rich blood clots, and neutrophil activation in the affected areas of the tissue samples.
"One striking detail was that these harmful effects only occurred in human blood vessels and not in the blood vessels of mice,"
notes first author PhD candidate Kawamura.
While the developed system helped to solve a long-standing mystery, the researchers believe that its potential is far beyond. With its potential for intravital imaging, it offers a powerful platform to study how other medicines might interact with the liver's delicate microvascular system. This could aid safer drug design and enable early detection of vascular injury—helping protect patients before symptoms become serious.
Reference
- Authors:
- Shuntaro Kawamura1, Yosuke Yoneyama1,2, Norikazu Saiki1,3, Yunheng Wu4, Chiharu Moriya2, Rio Ohmura1,3, Mari Maezawa1, Yoshihiro Shimada1, Yicheng Wang4, Kensaku Mori4,5,6, Noriki Okada7, Yasuharu Onishi7, Yukihiro Sanada7, Yuta Hirata7, Yasunaru Sakuma7 , and Takanori Takebe 1,2,3,8,9,10,11*
*Corresponding author - Title:
- Modeling antithymocyte globulin-induced microvasculopathy using human iPSC-derived vascularized liver organoids
- Journal:
- Cell Reports Medicin
- Affiliations:
- 1Human Biology Research Unit, Institute of Integrated Research, Institute of Science Tokyo, Japan
2Department of Genome Biology, Graduate School of Medicine, and Premium Research Institute for Human Metaverse Medicine (WPI-PRIMe), The University of Osaka, Japan
3Organoid Medicine Project, T-CiRA Joint Program, Japan
4Graduate School of Informatics, Nagoya University, Japan
5Information Technology Center, Nagoya University, Japan
6Research Center for Medical Bigdata, National Institute of Informatics, Japan
7Division of Gastroenterological, General and Transplant Surgery, Department of Surgery, Jichi Medical University, Japan
8Division of Gastroenterology, Hepatology and Nutrition & Division of Developmental Biology, Cincinnati Children’s Hospital Medical Center, USA
9The Center for Stem Cell and Organoid Medicine (CuSTOM), Cincinnati Children’s Hospital Medical Center, USA
10Department of Pediatrics, University of Cincinnati College of Medicine, USA
11Communication Design Center, Advanced Medical Research Center, Yokohama City University, Japan
Related articles
Further information
Professor Takanori Takebe
Human Biology Research Unit, Institute of Integrated Research, Institute of Science Tokyo
- ttakebe.ior@tmd.ac.jp
Contact
Public Relations Division, Institute of Science Tokyo
- Tel
- +81-3-5734-2975
- media@adm.isct.ac.jp